Scientists X-ray a Single Atom for the First Time
It took quantum tunneling and a particle accelerator to get the job done
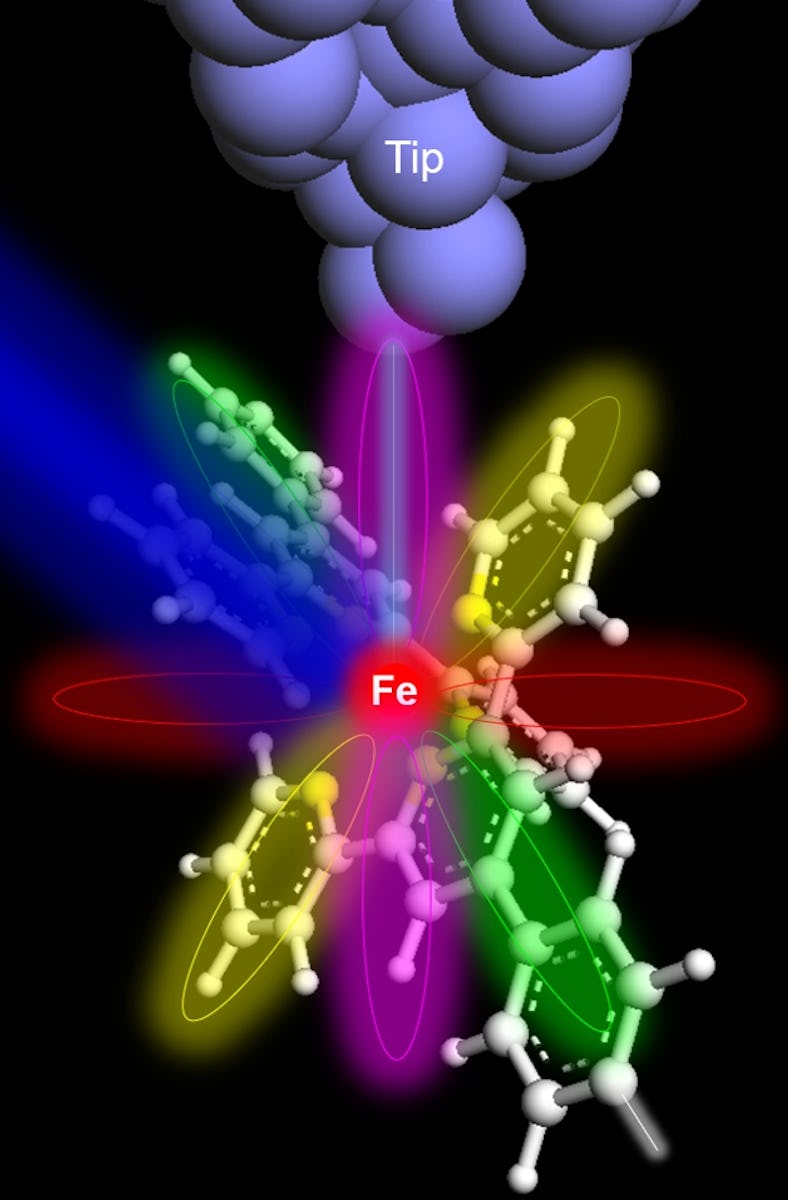
Ohio University physicist Saw Wai Hla and his colleagues were able to scan a single iron atom hidden amid a complex molecule, something that’s never been done with an X-ray before.
Extremely powerful microscopes can take images of individual atoms, and in fact they do it pretty much all the time, because we live in the future. But without X-rays and spectroscopy (a way of taking the “chemical fingerprint” of an object based on which wavelengths of light it absorbs or emits), images alone can’t tell scientists what element they’re looking at.
In recent experiments, Hla and his colleagues identified individual atoms and measured a few of their key properties. To do it, the researchers combined powerful, focused X-rays from a particle accelerator called a synchrotron with a technique called scanning tunneling microscopy, which uses a conductive tip to scan the surface of a sample. Their goal, Hla tells Inverse, was “to use X-ray spectroscopy at the ultimate limit of atomic scale. X-rays were discovered in 1895, almost 130 years ago, but have never been able to detect just one atom.”
They published their results in the journal Nature.
When X-rays from the synchrotron hit the iron atom in a complex structure of ring-shaped molecules, electrons from the iron atom quantum-tunnel to the tip of an instrument just half a nanometer away.
Fast Electrons and Bright Lights
A synchrotron, like the one at Argonne National Laboratory in Illinois, accelerates electrons to nearly the speed of light, then sends them zipping around a curved track. As the racing electrons take each curve of the track, they flash bright light — picture a tiny version of Tron. Equipment attached to the synchrotron splits the light into different wavelengths, sending the infrared light down one beamline and the X-ray light down another, for instance.
X-rays produced this way are brighter and more focused than the ones a normal X-ray machine can offer. Physicists can watch how light interacts with the molecules in a material to learn about really tiny details of its structure and makeup. But how tiny is tiny? If you want to know whether there’s iron in a particular material, for example, you’d better hope your sample contains at least a few thousand iron atoms, or the synchrotron X-ray will probably miss it.
But there’s good reason for materials scientists to want to detect individual atoms in much smaller samples.
“If one can detect the elemental and chemical state of an atom, then there will be a huge impact in many research areas,” says Hla.
There’s a single iron atom tucked into all of these interlocking molecular rings.
The Quantum Realm
That’s where quantum tunneling comes in handy. When the synchrotron X-rays hit a sample, they pump energy into the atoms. That new burst of energy riles up some of the electrons orbiting closest to the center of the atom, and they manage to break free.
Hla and his colleagues held the sharp metal tip of a scanning tunneling microscope instrument just half a nanometer away from the sample — close enough for a quantum tunnel to form, letting the newly-freed electrons travel from the sample into the instrument.
The electrons that arrive at the detector tip carry information about the atom they came from — such as which wavelengths of X-ray light the atom absorbed. Since each chemical element absorbs, reflects, and emits a specific set of light wavelengths, knowing which wavelengths the sample absorbed can reveal exactly which element an electron came from.
And in this case, Hla and his colleagues had just scanned the only iron atom in a large, complicated ring-shaped molecule. The scan also revealed how many electrons the iron atom was missing: two, in this case. That impacts how an atom can react with other atoms to form new chemical bonds, so it’s an important thing to know.
What’s Next
Hla and his colleagues repeated the experiment with a different big, complicated molecule, this time with a single atom of a rare-earth element called terbium hidden inside it. And once again, the detector identified the terbium atom and its chemical state.
Being able to combine detailed images with X-ray scans of a single atom of such a valuable element could be a huge help to engineers and materials scientists in the future.
“It might also be useful in medical research,” says Hla. “It will also have an impact on quantum information science, to name a few.” Next, Hla says he and his colleagues hope to measure the magnetic properties of a single atom, which will be useful for solid-state electronics.